Zebrafish
| Danio rerio | |
|---|---|
 |
|
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Actinopterygii |
| Order: | Cypriniformes |
| Family: | Cyprinidae |
| Genus: | Danio |
| Species: | D. rerio |
| Binomial name | |
| Danio rerio (Hamilton, 1822) |
|
| Synonyms | |
|
|
The zebrafish, Danio rerio, is a tropical freshwater fish belonging to the minnow family (Cyprinidae) of order Cypriniformes.[1] It is a popular aquarium fish, frequently sold under the trade name zebra danio, and is an important vertebrate model organism in scientific research.
Contents |
Taxonomy
The zebrafish are Cyprinidae with a derived member of the genus Danio. It has a sister group relationship with Danio kyathit.[2]
Zebrafish are closely related to the genus Devario, as demonstrated by a phylogenetic tree of close species. "Figure 2. Phylogenetic relationships among danios". http://www.nature.com/hdy/journal/v97/n3/fig_tab/6800867f2.html#figure-title.
Distribution
The zebrafish is native to the streams of the southeastern Himalayan region,[2] including the countries Pakistan, Bangladesh, Nepal, and Myanmar.[3] It arose in the Ganges region in Eastern India. It commonly inhabits streams, canals, ditches, ponds, and slow-moving to stagnant water bodies, including rice fields.[4]
Zebrafish have been introduced to parts of the United States, presumably by deliberate release or by escape from fish farms.[3] They have also been sighted in Colombia.
Description

The fish is named for the five uniform, pigmented, horizontal blue stripes on the side of the body, all of which extend to the end of the caudal fin. Its shape is fusiform and laterally compressed, with its mouth directed upwards. Males are torpedo-shaped and have gold stripes between the blue stripes; females have a larger, whitish belly and have silver stripes instead of gold. Adult females will exhibit a small genital papilla in front of the anal fin origin. The zebrafish can grow to 6.4 centimetres (2.5 in), although it is uncommon for them to grow past 4 cm in captivity. Life-span in captivity is around 2–3 years, although in ideal conditions, may extend to 5 years.[4]
Reproduction
The approximate generation time for the Danio is 3–4 months. A male must be present for ovulation and spawning to occur. Females are able to spawn at intervals of 2–3 days, laying hundreds of eggs in each clutch. Upon release, embryonic development begins; absent sperm, growth stops after the first few cell divisions. Fertilized eggs almost immediately become transparent, a characteristic that makes D. rerio a convenient research model species.[4] Development progresses rapidly. Precursors to all major organs appear within 36 hours of fertilization. Hatching takes place 48–72 hours after fertilization, depending on the embryo's internal conditions and the external temperature, ideally 28.5 °C (83.3 °F). Swimming and feeding behavior begin about 36 hours later. The sex of juveniles cannot be distinguished except by dissection, and sex determinants are not clearly understood.
Feeding
The zebrafish is omnivorous. It primarily eats zooplankton, insects and insect larvae, and phytoplankton. It can eat a variety of other foods, such as worms and small crustaceans if its preferred sources are not readily available.[4] Most Danios accept common food flakes and tubifex worms in the aquarium.
Strains
Recently, transgenic zebrafish have become commercially available that express green fluorescent protein, red fluorescent protein, and yellow fluorescent protein. They are tradenamed GloFish. Other subspecies include golden, sandy, longfin and leopard.
The leopard danio, previously known as Danio frankei, is a spotted colour morph of the zebrafish caused by a pigment mutation.[5] Xanthistic forms of both the zebra and leopard pattern, along with long-finned subspecies, have been obtained via selective breeding programs for the aquarium trade.[6]
Wild-type strains
The Zebra Fish Information Network[7] provides up-to-date information about current known wild-type (WT) strains of D. rerio. The most commonly found WT strains are: AB, TÜ, IN and WIK.
|
AB (AB) |
HK/AB (HK/AB) |
SJA (SJA) |
Hybrids
Hybrids between different Danio species may be fertile: e.g. between D. rerio and D. nigrofasciatus.[8]
See link for photos of hybrids: Figure 1. Danio pigment pattern diversity and phenotypes of D. rerio hybrids with other danios.
Aquarium care
Zebrafish are hardy fish and considered good for beginner aquarists. Their ease of keeping and breeding, beauty, price, playful nature and broad availability all contribute to their popularity. They thrive best in 22–28 °C (72–82 °F) water. They need an aquarium of 10 gallons or more, and they do well in schools. They also thrive as shoals of six or more, and interact well with other fish types in the aquarium. However, they are susceptible to Oodinium or velvet disease, microsporidia (Pseudoloma neurophilia), and Mycobacterium species. Given the opportunity, adults eat hatchlings, which may be protected by separating the two groups with a net, breeding box or separate tank.
In scientific research

D. rerio is a common and useful model organism for studies of vertebrate development and gene function.[2] George Streisinger at the University of Oregon established its utility. Success with it in large scale forward genetic screens (commonly referred to as the Tübingen/Boston screens) consolidated its importance. The scholarly journal Development[9] devoted an issue[10] to research using it in celebration of this landmark. It has a dedicated online database of genetic, genomic, and developmental information, the Zebrafish Information Network (ZFIN). D. rerio is one of the few fish species to have reached space. They may supplement higher vertebrate models, such as rats and mice.
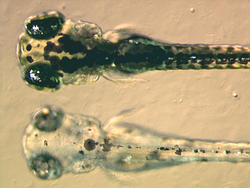
Research with D. rerio has allowed advances in the fields of developmental biology, oncology[12], toxicology[13], reproductive studies, teratology, genetics, neurobiology, environmental sciences, stem cell and regenerative medicine[14], and evolutionary theory[15].
Attractive characteristics
Its greatest advantages for use as a model system include:
- Fully-sequenced genetic code
- Well understood, easily observable and testable developmental behaviors
- Availability of well-characterized mutants
Other advantages:
- Rapid embryonic development (progressing from eggs to larvae in under three days, although overall generation time is comparable to that of mice)
- Large, robust, and transparent embryos that develop outside the mother[16]
- Nearly constant size during early development facilitates simple staining techniques
- Drugs may be administered by adding directly to the tank.
- Unfertilized eggs can be made to divide
- Two-celled embryo can be fused into a single cell, creating a homozygous embryo
Regeneration
Zebrafish have the ability to regenerate fins, skin, the heart[17] and the brain (in larval stages). Heart muscle regeneration does not make use of stem cells; instead, mature heart muscle cells regress to a stem-cell-like state and redifferentiate.[17]
Zebrafish have also been found to regenerate photoreceptor cells and retinal neurons following injury. The mechanisms of this regeneration are unknown. Researchers frequently amputate the dorsal and ventral tail fins and analyze their regrowth to test for mutations. This research is leading the scientific community in the understanding of healing/repair mechanisms in vertebrates. It has also been found if the same fin is damaged enough times, the fish will grow a new fin which will have mutated only a small amount. Most scientists believe this is a defence mechanism to try to prevent the fin from being damaged again.
Genetics
Gene expression
A common reverse genetics technique is to reduce gene expression or modify splicing using Morpholino antisense technology. Morpholino oligonucleotides (MO) are stable, synthetic macromolecules that contain the same bases as DNA or RNA; by binding to complementary RNA sequences, they reduce the expression of specific genes. The journal Genesis[18] devoted an issue[19] to research using MO, mostly in D. rerio. MO can be injected into one cell of an embryo after the 32-cell stage, reducing gene expression in only cells descended from that cell. However, cells in the early embryo (less than 32 cells) are interpermeable to large molecules,[20][21] allowing diffusion between cells. A known problem with gene knockdowns is that, because the genome underwent a duplication after the divergence of ray-finned fishes and lobe-finned fishes, it is not always easy to silence the activity one of the two gene paralogs reliably due to complementation by the other paralog.
Despite the complications of the zebrafish genome, a number of commercially available global platforms for analysis of both gene expression by microarrays and promoter regulation using ChIP-on-chip exist.
Gene sequencing
In 2009, researchers at the Institute of Genomics and Integrative Biology, Delhi announced the sequencing of a wild caught strain, containing 1.7 billion genetic letters.[22][23]
Mitochondrial DNA
In October 2001, researchers from the University of Oklahoma published D. rerio's complete mitochondrial DNA sequence.[24] Its length is 16,596 base pairs. This is within 100 base pairs of other related species of fish, and it is notably only 18 pairs longer than the goldfish (Carassius auratus) and 21 longer than the carp (Cyprinus carpio). Its gene order and content is identical to the common vertebrate form of mitochondrial DNA. It contains 13 protein-coding genes and a noncoding control region containing the origin of replication for the heavy strand. In between a grouping of five tRNA genes, a sequence resembling vertebrate origin of light strand replication is found. It is difficult to draw evolutionary conclusions because it is difficult to determine whether base pair changes have adaptive significance via comparisons with other vertebrates nucleotide sequences.
Pigmentation gene
In December 2005, a study of the golden strain identified the gene responsible for its unusual pigmentation as SLC24A5, a solute carrier that appeared to be required for melanin production, and confirmed its function with a Morpholino knockdown. The orthologous gene was then characterized in humans and a one base pair difference was found to strongly segregate fair-skinned Europeans and dark-skinned Africans.[25]
Transgenesis
Transgenesis is a popular approach to study the function of genes in zebrafish. Construction of transgenic zebrafish is rather easy by a method using the Tol2 transposon system. [26]
In environmental monitoring
Estrogen detection
In January 2007, Chinese researchers at Fudan University genetically modified fish to detect estrogen pollution in lakes and rivers, which is linked to male infertility.[27]
In medical research
Repairing retinal damage
In 2007, researchers at University College London grew a type of zebrafish adult stem cell found in the eyes of fish and mammals that develops into neurons in the retina—the part of the eye that sends messages to the brain. These cells could be injected in the eye to treat diseases that damage retinal neurons—nearly every disease of the eye, including macular degeneration, glaucoma, and diabetes-related blindness. Retinal damage is responsible for most cases of sight loss. The researchers studied Müller glial cells in the eyes of humans aged from 18 months to 91 years and were able to develop them into all types of retinal neurons. They were able to grow them easily in the lab. The stem cells successfully migrated into diseased rats' retinas and took on the characteristics of the surrounding neurons. The team is working on the same approach in humans.[28]
Transparent adult bodies
In 2008, researchers at Children's Hospital Boston developed a new strain of zebrafish, named Casper, whose adult bodies were transparent.[29] This allows for detailed visualization of cellular activity, circulation, metastasis and many other phenomena. Because many gene functions are shared between fish and humans, Casper is expected to yield insight into human diseases such as leukemia and other cancers.[29][30]
See also
- The subfamily Danionin
- Development of fish: post-fertilization development and axes formation Regional_specification#Fish
- List of freshwater aquarium fish species
- See-through_frog
References
- ↑ Froese, R. and D. Pauly. Editors.. "Danio rerio". FishBase. http://www.fishbase.org/Summary/speciesSummary.php?ID=4653&genusname=Danio&speciesname=rerio. Retrieved 2007-04-07.
- ↑ 2.0 2.1 2.2 Mayden, Richard L.; Tang, Kevin L.; Conway, Kevin W.; Freyhof, Jörg; Chamberlain, Sarah; Haskins, Miranda; Schneider, Leah; Sudkamp, Mitchell; Wood Robert M.; Agnew, Mary; Bufalino, Angelo; Sulaiman, Zohrah; Miya, Masaki; Saitoh, Kenji; He, Shunping (2007). "Phylogenetic relationships of Danio within the order Cypriniformes: a framework for comparative and evolutionary studies of a model species". J. Exp. Zool. (Mol. Dev. Evol.) 308B (5): 642–654. doi:10.1002/jez.b.21175. PMID 17554749.
- ↑ 3.0 3.1 USGS NAS - Nonindigenous Aquatic Species
- ↑ 4.0 4.1 4.2 4.3 Spence R, Gerlach G, Lawrence C, Smith C (February 2008). "The behaviour and ecology of the zebrafish, Danio rerio". Biological Reviews 83 (1): 13–34. doi:10.1111/j.1469-185X.2007.00030.x. PMID 18093234.
- ↑ Watanabe M, Iwashita M, Ishii M, et al. (September 2006). "Spot pattern of leopard Danio is caused by mutation in the zebrafish connexin41.8 gene". EMBO Rep. 7 (9): 893–7. doi:10.1038/sj.embor.7400757. PMID 16845369.
- ↑ Mills, Dick (1993). Eyewitness Hnbk Aquarium Fish. Harper Collins. ISBN 0-7322-5012-9.
- ↑ ZFIN
- ↑ Parichy, DM (September 2006). "Evolution of danio pigment pattern development". Heredity 97 (3): 200–210. doi:10.1038/sj.hdy.6800867. PMID 16835593. http://www.nature.com/hdy/journal/v97/n3/full/6800867a.html.
- ↑ Development journal
- ↑ "Zebrafish issue". Development 123 (1). December 1996. http://dev.biologists.org/content/vol123/issue1/.
- ↑ See link for pigmentation mutants of D rerio: Figure 5. Pigment pattern mutants within D. rerio. Mutant names are shown along with gene identities in parentheses when known. For example, the picasso phenotype results from mutations in errb3
- ↑ Xiang J, et al. (February 2009). "Identifying Tumor Cell Growth Inhibitors by Combinatorial Chemistry and Zebrafish Assays". PLoS ONE 4 (2): e4361. doi:10.1371/journal.pone.0004361. PMID 19194508.
- ↑ Hill AJ, Teraoka H, Heideman W & Peterson RE (July 2005). "Zebrafish as a Model Vertebrate for Investigating Chemical Toxicity". Toxicological Sciences 86 (1): 6–19. doi:10.1093/toxsci/kfi110. PMID 15703261. http://toxsci.oxfordjournals.org/cgi/content/full/86/1/6.
- ↑ Major RJ, Poss KD (2007). "Zebrafish heart regeneration as a model for cardiac tissue repair". Drug Discov Today Dis Models 4 (4): 219–225. doi:10.1016/j.ddmod.2007.09.002. PMID 19081827.
- ↑ Parichy, DM (September 2006). "Evolution of danio pigment pattern development". Heredity 97 (3): 200–210. doi:10.1038/sj.hdy.6800867. PMID 16835593. http://www.nature.com/hdy/journal/v97/n3/full/6800867a.html.
- ↑ Dahm, Ralf (2006). "The Zebrafish Exposed". American Scientist 94 (5): 446–453
- ↑ 17.0 17.1 Wade, Nicholas (March 24, 2010). "Research Offers Clue Into How Hearts Can Regenerate in Some Species". New York Times.
- ↑ genesis - The Journal of Genetics and Development
- ↑ "Zebrafish issue". Genesis 30 (3). July 2001. http://www3.interscience.wiley.com/cgi-bin/jissue/85006212.
- ↑ Kimmel CB, Law RD (March 1985). "Cell lineage of zebrafish blastomeres. I. Cleavage pattern and cytoplasmic bridges between cells". Dev Biol. 108 (1): 78–85. doi:10.1016/0012-1606(85)90010-7. PMID 3972182. http://linkinghub.elsevier.com/retrieve/pii/0012-1606(85)90010-7.
- ↑ Kimmel CB, Law RD (March 1985). "Cell lineage of zebrafish blastomeres. III. Clonal analyses of the blastula and gastrula stages". Dev Biol. 108 (1): 94–101. doi:10.1016/0012-1606(85)90012-0. PMID 3972184. http://linkinghub.elsevier.com/retrieve/pii/0012-1606(85)90012-0.
- ↑ Decoding the Genome Mystery Indian Express, July 5, 2009.
- ↑ Genome@IGIB Institute of Genomics and Integrative Biology (IGIB)
- ↑ Broughton RE, Milam JE, Roe BA (October 2001). "The complete sequence of the zebrafish (Danio rerio) mitochondrial genome and evolutionary patterns in vertebrate mitochondrial DNA". Genome Res 11 (11): 1958–67. doi:10.1101/gr.156801. PMID 11691861. PMC 311132. http://genome.cshlp.org/content/11/11/1958.full.
- ↑ Lamason RL, Mohideen MA, Mest JR, et al. (December 2005). "SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans". Science 310 (5755): 1782–6. doi:10.1126/science.1116238. PMID 16357253.
- ↑ Kawakami, K, et al. (2004). "A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish". Developmental Cell 7 (1): 133–144. doi:10.1016/j.devcel.2004.06.005. PMID 15239961.
- ↑ Song Houyan and Zhong Tao, professors at Fudan's molecular medicine lab, spent three years cloning estrogen-sensitive genes and injecting them into the fertile eggs of zebrafish. The modified fish turn green if they are placed into water that is polluted by estrogen. Fudan scientists turn fish into estrogen alerts
- ↑ Zebra fish may point way to cure for blindness The China Post Friday, August 3, 2007.
- ↑ 29.0 29.1 White RM, Sessa A, Burke C, et al. (February 2008). "Transparent adult zebrafish as a tool for in vivo transplantation analysis". Cell Stem Cell 2 (2): 183–9. doi:10.1016/j.stem.2007.11.002. PMID 18371439.
- ↑ livescience.com
Further reading
- "Danio rerio". Integrated Taxonomic Information System. http://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=163699. Retrieved 12 November 2004.
- Zebrafish on FishBase.
- Lambert, Derek J (1997). Freshwater Aquarium Fish. Edison, New Jersey: Chartwell Books. pp. 19. ISBN 0-7858-0867-1.
- Sharpe, Shirlie. "Zebra Danio". Your Guide to Freshwater Aquariums. http://freshaquarium.about.com/cs/cyprinids2/p/zebradanio.htm. Retrieved December 15, 2004.
- Kocher TD, Jeffery WR, Parichy DM, Peichel CL, Streelman JT, Thorgaard GH (2005). "Special feature--roundtable discussion. Fish models for studying adaptive evolution and speciation". Zebrafish 2 (3): 147–56. doi:10.1089/zeb.2005.2.147. PMID 18248189.
- The Zebrafish Information Network (ZFIN)
- Bradbury J (2004). "Small Fish, Big Science". PLoS Biology 2 (5): 568–72. doi:10.1371/journal.pbio.0020148. PMID 15138510.
External links
- The Zebrafish Information Network (ZFIN)
- The Zebrafish International Resource Center (ZIRC)
- The Zebrafish Genome Sequencing Project (Wellcome Trust Sanger Institute)
- FishMap : The Zebrafish Community Genomics Browser
- Zebrafish GenomeWiki Beta Preview maintained at the Institute of Genomics and Integrative Biology
- The Zebrafish wild-type strain Genome sequencing initiative at the Institute of Genomics and Integrative Biology
- Danio rerio
- Danio rerio embryonic development images
- Sanger Institute Zebrafish Mutation Resource
- Heartbeat and blood flow in transgenic Zebrafish - movies
|
|||||